Research
Our research is primarily focused on signaling by Rho of Plants (ROP) small GTPases and their effectors. ROPs regulate polar cell growth as well as abiotic and biotic stress responses and are thus excellent candidates for studying the interaction between developmental and stress signaling. We study both ROP-regulated cell polarity and the function of ROPs and their effectors in the regulation of abiotic stress responses. We are using Arabidopsis and tomato as model plant systems in combination with classical and state-of-the-art technologies including genome editing, genomics and molecular biology, imaging, biochemical analysis, and physiological analysis at the organ and whole plant levels.
ROPs and stress signaling
The reduction in freshwater resources is becoming a widespread problem due to global warming. Therefore, the development of crops with increased water use efficiency and improved drought tolerance is of the highest priority. Using genome editing we have generated non-transgenic tomato mutants which display increased water use efficiency and drought tolerance. Remarkably, large-scale field experiments showed that the fruit quantity and quality of the mutant plants are not affected. We are currently studying the molecular basis for the increased water use efficiency and drought tolerance of the mutant plants and the applicability of similar mutations in other plant species. In parallel, we are working towards commercializing the mutant plants for agricultural use.

ROPs and cell polarity
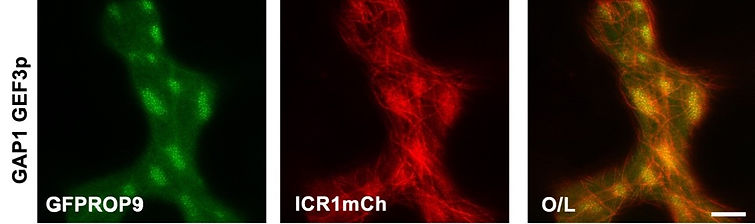
ROPs form self-organizing polarity domains when coexpressed with their activator ROP Guanyl nucleotide Exchange Factor (ROPGEF) and inhibitor ROP GTPase Activating protein (ROPGAP). We discovered that in the domains ROPs become immobile, that domain formation depends on interaction with anionic lipids, and that it is independent of RHOGDI-mediated recycling. In the domains, ROPs differ in their ability to interact with effectors. We would like to understand how ROPs become immobile and the molecular consequences of their differential activities in the domains.
We discovered that in addition to GDP/GTP exchange, ROP activation involves transient S-acylation on conserved G-domain cysteine residues which causes their partitioning into lipid microdomains and is required for their function. We would like to understand how transient S-acylation affects ROP function during development and in the regulation of abiotic stress signaling.
To enhance our understanding of ROP signaling, we have developed novel ROP activation probes and we use them for analyzing ROP activation status in cell and tissue-specific fashion.


The ICR family of ROP interacting proteins
We discovered a family of ROP interacting proteins which we named Interactor of Constitutively active ROP (ICRs). The ICRs are microtubule-associated coiled-coil domain proteins. The ICRs are conserved in higher plants and have two signature motifs: a C-terminal QRKAA domain which is required for interaction with GTP bound ROPs and an N-terminal QEEL domain. The ICRs function as scaffolds which interact with specific proteins and via interaction with ROPs and microtubules regulate protein function by recruiting them to specific cellular domains. We are studying the function of the ICRs in development and stress signaling by combining genome editing, cell biology, bioinformatics, biochemichal physiological methodologies.
The function of osmotic stress and ABA in root development and tissue patterning
We discovered that ABA induces earlier maturation of the root which is associated with increased expression of the protoxylem-inducing transcriptional regulator VND7. We further showed that osmotic stress induces in an ABA-dependent fashion repatterning of root primary xylem tissue by increasing the expression of miRNA165/166 and consequential downregulation of HD-ZIPIII transcription factors in the stele. Our studies also showed that these functions of ABA are auxin independent.
Our studies also demonstrated that ABA-induced lateral root arrest takes place via lateral root endodermal initials and is associated with increased miR165 expression in the future quiescent center and a decrease in HD-ZIPII expression.
We are currently studying how ROP signaling and ABA are integrated with the regulation of root development.






